Oral graft-versus-host disease
The aim of this e-learning tool is to present readers with information on the epidemiology, clinical characteristics, diagnosis and management of oral graft-versus-host disease.
Graft-versus-host disease (GVHD) is an immunologic condition which develops following allogeneic hematopoietic stem cell transplantation (HSCT). It is a major cause of non-relapse morbidity and mortality in this group of patients. The incidence and prevalence of GVHD are increasing due to the extension of clinical indications for HSCT treatment and more prolonged patient survival and follow-up. GVHD is an indicator of treatment success because due to graft-versus-tumor (GVT) effect, there is a lower risk of malignancy relapse. Therefore, there is a balance between GVT and chronic GVHD for optimal transplantation results
Epidemiology
GVHD develops in 40-60% of patients following allogeneic hematopoietic stem cell transplantation (HSCT). The incidence is higher in pediatric patients. It is a major cause of morbidity and is fatal in approximately 15% of patients. Both acute (aGVHD) and chronic GVHD (cGVHD) may have oral manifestations. The incidence of chronic GVHD is varies from 25-80%.
Oral lesions are rarely seen in acute GVHD, and the incidence is therefore, unknown. In over 70% of patients with cGVHD, the oral cavity is affected.
Clinical Presentation
GVHD is a multisystem disease with acute and chronic Acute GVHD may be classified as classic (which occurs in the first 100 days after HSCT) and late, recurrent or persistent (if it occurs after this period) which may be seen in patients on non-myeloablative conditioning. Likewise, cGVHD may also manifest as classic cGVHD (without overlapping but with some features of aGVHD) or as an overlap syndrome (with distinctions of both acute and chronic GVHD at the same time, for example in patients who receive donor lymphocyte infusions).
» Acute GVHD
Acute GVHD
- Acute GVHD primarily affects the skin, liver and the gastrointestinal (GI) tract.
- Skin lesions are often the earliest visible sign of the disease (14).
- The oral mucosa is rarely involved in aGVHD and the lesions are non-specific (15).
- Lesions may also be visible on the oral mucosa or lips, as gingivitis, mucositis, erythema or ulceration.
- In the case of overlap syndrome, oral lesions may be seen as lichen planus-like hyperkeratotic changes.
» View less
» Chronic GVHD
Chronic GVHD
- Chronic GVHD can manifest as one of three models: 1) de novo onset in a patient who did not have previous aGVHD lesions; 2) quiescent onset after the lesions of aGVHD have completely resolved; and 3) progressive onset when cGVHD immediately follows aGVHD.
- The disease can affect one or multiple organs. The skin, eyes, oral cavity, GI tract, liver and lungs are among the preferred sites involved.
» View less
» Oral Cavity
Oral Cavity
In the oral cavity, cGVHD can be classified as oral mucosal cGVHD, salivary gland disease and sclerotic disease.
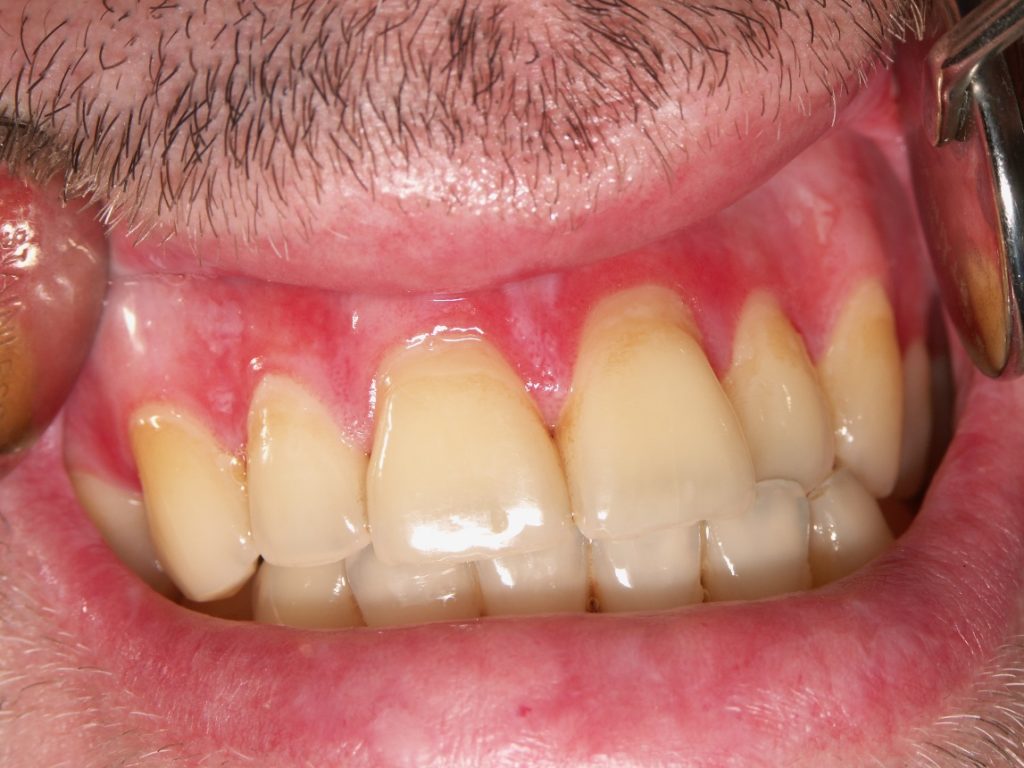
- Oral mucosal disease presents with white striae, erythema and ulcers, most frequently affecting buccal mucosa and lateral tongue (Figure 1).
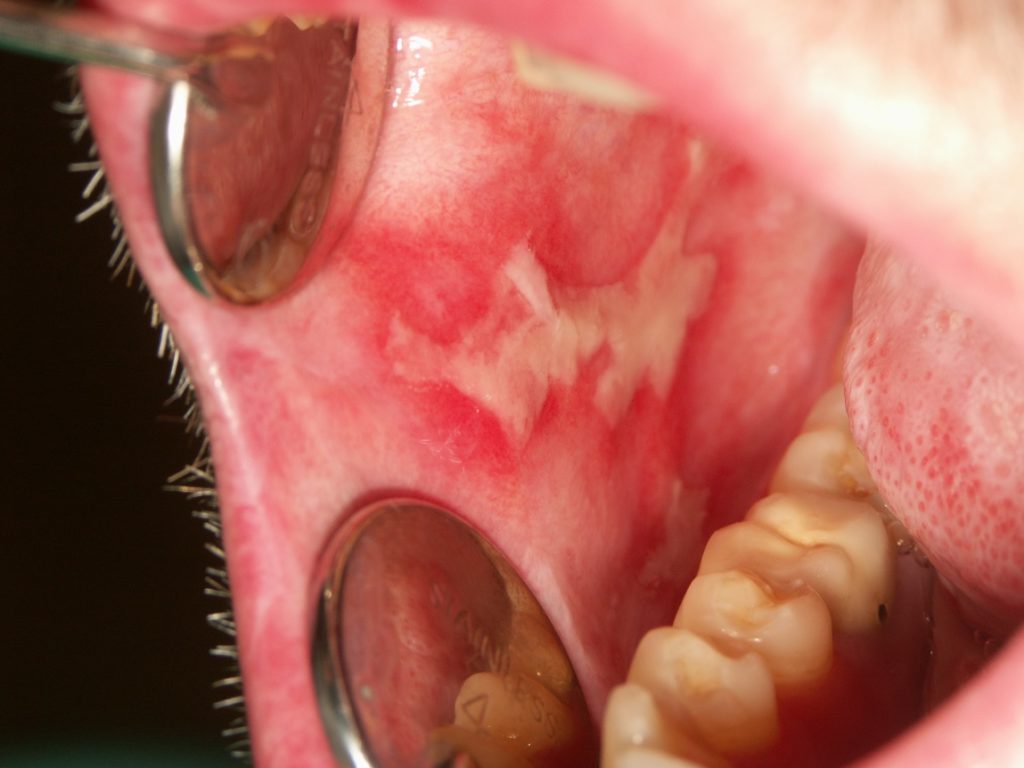
- The lesions are painful and limit food and beverage intake. In the gingiva, the lesions manifest as erythema and desquamation, with or without the presence of white striations (Figure 2).
- Gingival lesions are also painful and may impede oral hygiene.
- The sclerotic form of cGVHD affecting facial skin and the oral cavity, may manifest with restriction of mouth opening and loss of elasticity of the lips and restricted tongue movements.
- Severe fibrosis may result in shallow vestibules and periodontal defects which greatly deteriorates oral function. Oral hygiene and dental procedures can be severely compromised.
Assessment of oral cGVHD is important for clinical practice and clinical research.
» View less
» Diagnosis
Diagnosis
- The diagnosis of acute GVHD is established based on the history and clinical examination with skin, liver and gastrointestinal tract involvement within the first 100 days of HSCT and not meeting the dagnostic criteria for cGVHD.
- The diagnosis is based on the exclusion of other conditions and the coexistence of aGVHD in other organs (skin, liver, intestines) (15). These patients are classified as classic acute GVHD.
- Some patients may develop signs of aGVHD beyond 100 days of HSCT without diagnostic signs of cGVHD. These patients are classified as persistent, recurrent or late aGVHD.
Patients with at least one distinctive sign of cGVHD without signs of aGVHD are classified as classic cGVHD. Patients with signs of both aGVHD and cGVHD are classified as overlap syndrome (23). Diagnostic distinction between aGVHD and cGVHD is presented in Figure 3.
Table: Acute and chronic GVHD
| Acute GVHD | Chronic |
| Rash Nausea and Vomiting Loss of appetite Diarrhoea Cholestatic hepatitis | Typical lichenoid features Erythema Ulceration Leukoplakia Dry mouth Limited mouth opening |
| Classic: Occurs within 100 days | Classic: at least one diagnostic / distinctive manifestation, no time restriction |
| Persistent / Recurrent / Late acute: Beyond 100 days | Overlap syndrome: Features of acute and chronic, no time restriction |
- If the lesions of oral cGVHD have a typical lichenoid presentation, the diagnosis is established clinically.
- In cases presenting as erythema, ulceration or as a white plaque, with an absence of typical white striations, a biopsy is needed to establish the diagnosis and to exclude other conditions.
» View less
Differential Diagnoses
The differential diagnosis of oral GVHD may include viral and fungal infections (usually HSV, candidosis), erythema multiforme, lichen planus, lichenoid reactions and discoid lupus. Histopathological findings of oral mucosal cGVHD include lichenoid interface inflammation, leukocyte exocytosis and keratinocyte apoptosis. Histopathological findings of salivary gland cGVHD demonstrates intralobular periductal lymphocytic infiltration (frequently seen with fibrosis) and exocytosis of lymphocytes into intralobular ducts and acini.
Management
The aim of oral GVHD treatment is to alleviate symptoms to enable uninterrupted oral function. Topical treatment is the same for acute and chronic GVHD. In cases of cGVHD with oral cavity involvement, topical therapy is the treatment of choice. Topical therapy may be an adjunct to systemic therapy. The treatment of choice as systemic therapy included corticosteroids with or without the use of cyclosporine.
» Mucosal cGVHD
Mucosal cGVHD
- First-line treatment of oral mucosal cGVHD lesions include topical corticosteroids and calcineurin inhibitors such as tacrolimus.
- Topical corticosteroids of varying potency can be applied as solutions, gels, creams or ointments several times a day. Intralesional injections of corticosteroids (e.g. triamcinolone acetonide) for localized ulceration is helpful for persistent lesions resistant to treatment.
- Topical tacrolimus may be used as add-on therapy. Tacrolimus ointment (available as 0.03% and 0.1%) is recommended for lesions involving the lips, while tacrolimus solution is a better choice for intraoral involvement .
- Oral pain can be controlled with topical anesthetic agents such as viscous lidocaine, or systemic analgesics, including opioids.
- Maintaining oral hygiene is difficult. Patients should be advised to use neutral-pH toothpastes and to avoid mint flavouring which may act as an irritant. Ultra-soft toothbrushes are recommended in the case of painful oral lesions.
» View less
» Sclerotic oral cGVHD
Sclerotic oral cGVHD
- Sclerotic changes tend to be progressive and non-reversible.
- Patients should be instructed to undertake physiotherapy exercises daily to enhance flexibility of the perioral tissues.
- Systemic cGVHD treatment may be warranted, and in some cases, surgical treatment may be necessary.
» View less
Complications
- Patients frequently develop oral candidosis due to dry mouth, immunosuppression and antimicrobial prophylaxis. Prophylactic antifungal therapy should be given to patients with predisposing factors. In cases where oral candidosis develops, it should be treated with systemic antifungal therapy, usually fluconazole.
- Recurrent HSV infections are also possible, often with atypical presentations. Systemic antivirals (acyclovir) are the treatment of choice.
- After HSCT, patients have an increased risk of a second primary cancer. The risk for oral cancer in cGVHD patients is 6 times higher compared to the general population. The longer the time since transplantation, the risk of cancer arising increases. The average time for oral cancer development following HSCT is 6-8 years. NIH recommendations suggest patients are screened twice a year. In cases of oral cancer development, the follow-up time should be prolonged due to risk of recurrence.
Summary
Most patients with cGVHD present with oral manifestations of the disease. Treatment is symptomatic and aimed at preserving function and improving quality of life. Due to the increased risk for dental caries development and risk of oral cancer, long-term follow-up and screening of oral mucosa twice a year is recommended.
References and Further Reading
Treister N, Schubert MS, Fall-Dickson JM. Oral chronic graft vs host disease. In: Vogelsang GB, Pavletic SZ, eds. Chronic Graft-Versus-Host Disease: Interdisciplinary Management. New York: Cambridge University; 2009:182–198.
Mays JW, Fassil H, Edwards DA, Pavletic SZ, Bassim CW. Oral chronic graftversus-host disease: current pathogenesis, therapy, and research. Oral Dis. 2013;19(4):327–346.
Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012;119(1):296–307.
Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood 1990;75(12):2459–64.
Kuten-Shorrer M, Woo SB, Treister NS. Oral graft-versus-host disease. Dent Clin North Am. 2014;58(2):351-68.
Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol 2012;12(6):443–58.
Weiden PL, Sullivan KM, Flournoy N, et al. Antileukemic effect of chronic graftversus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med 1981;304(25):1529–33.
Schubert MM, Correa ME. Oral graft-versus-host disease. Dent Clin North Am 2008;52(1):79–109 viii-ix.
Treister NS, Cook EF Jr, Antin J, et al. Clinical evaluation of oral chronic graftversus-host disease. Biol Blood Marrow Transplant 2008;14(1):110–5.
Treister N, Duncan C, Cutler C, et al. How we treat oral chronic graft-versus-host disease. Blood 2012;120(17):3407–18.
Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21(3):389–401.e1.
Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graftversus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11(12):945–56.
Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood 2003;102(2):756–62.
Elad S, Aljitawi O, Zadik Y. Oral graft-versus-host disease: a pictorial review and a guide for dental practitioners. Int Dent J. 2020 Jun 28. doi: 10.1111/idj.12584.
Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15(6):825–8.
Dignan FL, Clark A, Amrolia P, et al. Diagnosis and management of acute graftversus-host disease. Br J Haematol 2012;158(1):30–45.
Shulman HM, Sale GE, Lerner KG, et al. Chronic cutaneous graft-versus-host disease in man. Am J Pathol 1978;91(3):545–70.
Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant 2003;9(4):215–33.
Imanguli MM, Alevizos I, Brown R, et al. Oral graft-versus-host disease. Oral Dis 2008;14(5):396–412.
Meier JK, Wolff D, Pavletic S, et al. Oral chronic graft-versus-host disease: report from the International Consensus Conference on Clinical Practice in cGVHD. Clin Oral Investig 2011;15(2):127–39.
Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow. Transplantation 2006;12(3):252–66.
Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transpl 2015;21(6):984–99.
Margaix-Muñoz M, Bagán JV, Jiménez Y, Sarrión MG, Poveda-Roda R. Graft-versus-host disease affecting oral cavity. A review. J Clin Exp Dent. 2015;7(1):e138-45.
Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology working group report. Biol Blood Marrow Transplant 2006;12(1):31–47.
Fall-Dickson JM, Cordes S, Berger AM. Oral complications. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 11th ed. Philadelphia: Wolters Kluwer; 2019:2094–2108.
Jaglowski SM, Blazar BR. How ibrutinib, a B-cell malignancy drug, became an FDA-approved second-line therapy for steroid-resistant chronic GVHD. Blood Adv. 2018;2(15):2012–2019.
Fall-Dickson JM, Pavletic SZ, Mays JW, Schubert MM. Oral Complications of Chronic Graft-Versus-Host Disease. J Natl Cancer Inst Monogr. 2019;2019(53): lgz007.
Treister N, Li S, Lerman MA, et al. Narrow-band UVB phototherapy for management of oral chronic graft-versus-host disease. Photodermatol Photoimmunol Photomed 2015;31(2):75–82.
Elad S, Jensen SB, Raber-Durlacher JE, et al. Clinical approach in the management of oral chronic graft-versus-host disease (cGVHD) in a series of specialized medical centers. Support Care Cancer 2015;23(6):1615–22.
Carpenter PA, Kitko CL, Elad S, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transpl 2015;21(7):1167–87.
Elad S, Zadik Y, Zeevi I, et al. Oral cancer in patients after hematopoietic stem-cell transplantation: long-term follow-up suggests an increased risk for recurrence. Transplantation 2010;90(11):1243–4.







