Leukoplakia
Several definitions have been suggested for oral leukoplakia (OL) and this has continuously evolved over the last 40 years (Table 1). The most recent and widely accepted definition was proposed by WHO Collaborating Centre for Oral Cancer in 2007 and re-confirmed in 2020. Leukoplakia is defined as “A predominantly white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer”.
The term leukoplakia is used when any other condition of the oral mucosa that may present as a white lesion has been excluded (e.g. frictional keratosis, lichen planus, white sponge nevus, hairy leukoplakia etc.). By definition leukoplakia is a clinical entity, diagnosis by exclusion and it does not have a specific histopathological component.
Epidemiology and Risk Factors for Development of Oral Leukoplakia
Epidemiology:
- Commonly diagnosed in middle-aged to elderly patients.
- More commonly identified in males.
- OL is six times more common among smokers than non-smokers.
- The prevalence of OL varies significantly with geographical variations due to different risk factors, such as betel nut chewing in South East Asia .
Risk Factors:
- OL is associated with various risk factors, similar to those observed in oral cancer, such as tobacco (both smoked and smokeless), heavy alcohol consumption, betel quid chewing (especially in South Asian countries) and, for lesions involving the lip, ultraviolet (UV) light exposure
- Alcohol consumption is an independent risk factor. It has been established that these factors may have an aetiological role in the development of OL in more than 75% of the affected individuals.
Clinical Presentation
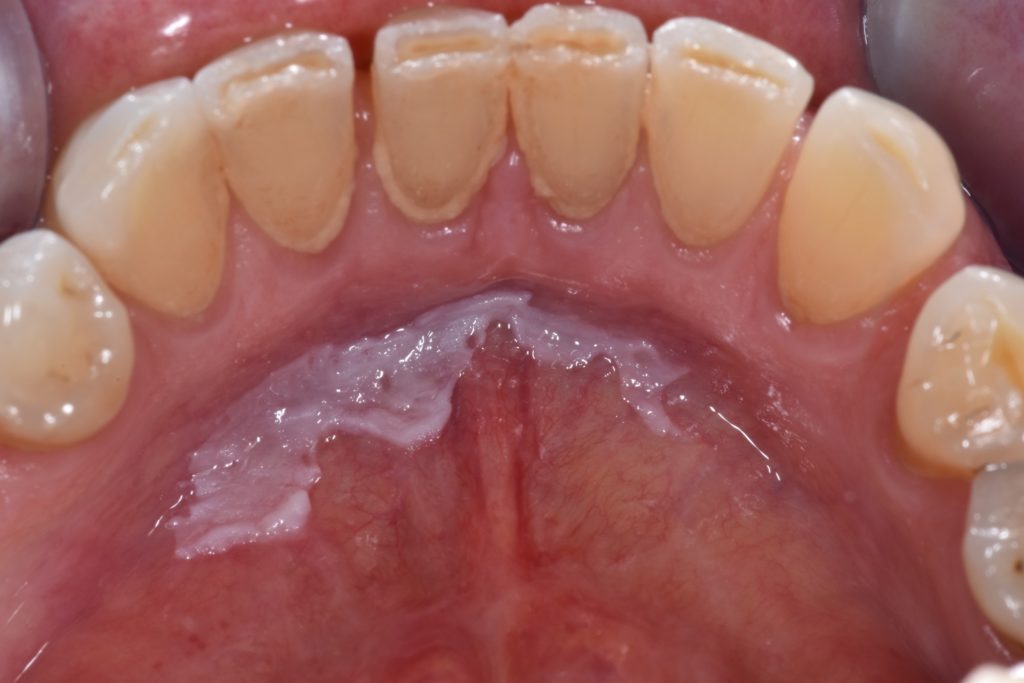
- OL may arise anywhere in the oral cavity and may be uni- or multifocal. However, in Western industrialized populations, the most common sites of involvement are the lateral border of the tongue and floor of the mouth.
- In Asian populations, due to the frequent consumption of betel quid, the buccal mucosa and the labial mucosa are often affected.
- Clinically, leukoplakia show a wide range of variable features in appearance (white, red, and mixed white and red, plaque/plateau granular, atrophic) texture (smooth, corrugated, verrucous granular, soft, firm or hard), and size and shape.
- The clinical extension of oral leukoplakia may vary in size from a relatively small and well circumscribed lesion to extensive involving a large area of the oral mucosa.
Leukoplakia can be clinically sub-classified into two types: homogenous and non-homogenous. Figures 1 & 2.
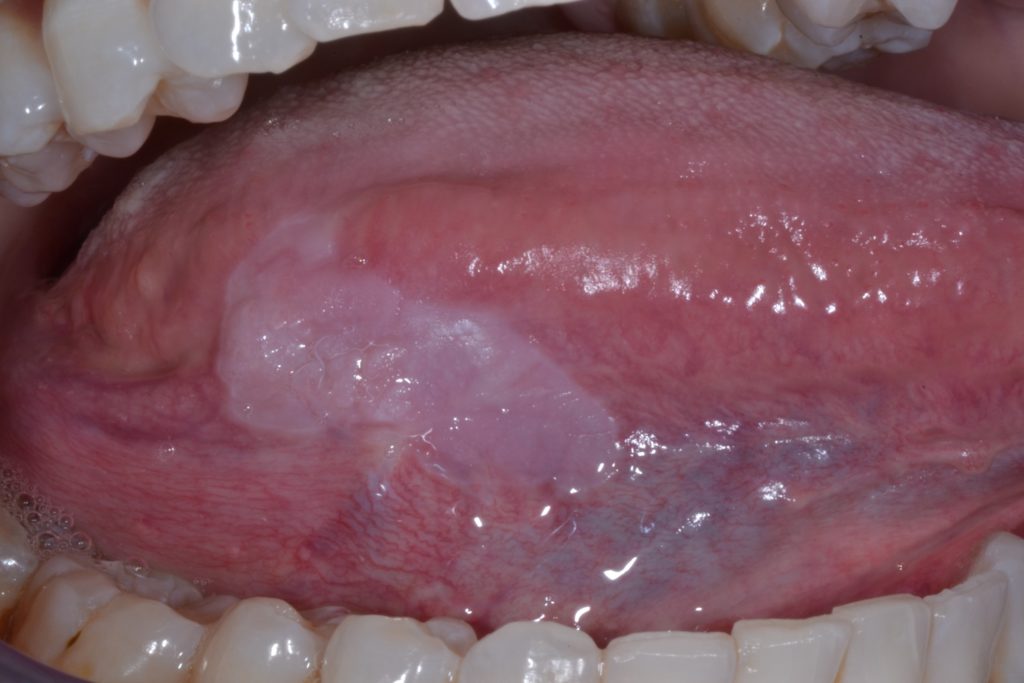
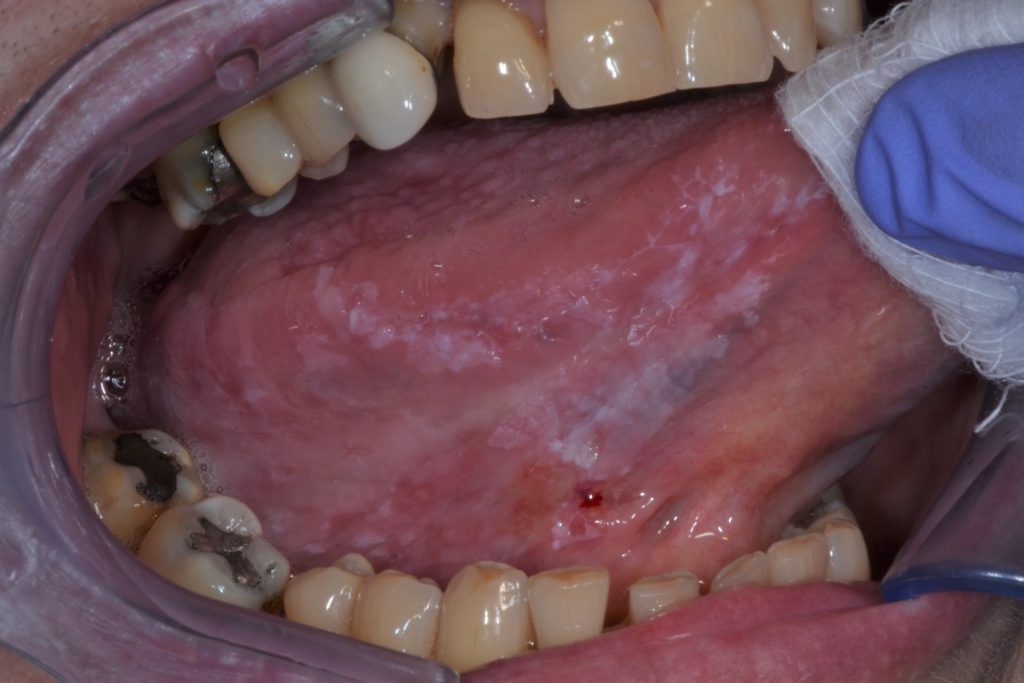
This distinction is purely clinical, based on distinct features such as surface, colour and texture: homogenous leukoplakia and non-homogenous leukoplakia (Table 2).
| Oral Leukoplakia | |
| Homogenous leukoplakia | Non-homogenous leukoplakia |
| Uniform flat and thin white plaque/patch with well-defined margins, smooth surface associated with shallow cracks/fissures. Cannot be scraped off. | Speckled: mixed white and red colour Nodular: small polypoid outgrowths, rounded, red or white excrescences Verrucous or exophytic: wrinkled or corrugated surface. Cannot be scraped off. |
Table 2: Classification of the oral leukoplakia based on the distinct clinical features
Homogenous Leukoplakia:
- Characterized by a uniform flat and thin white plaque/patch with well-defined margins and smooth surface which may be associated with shallow cracks/fissures of the surface keratin. Further illustrated in Figure 3.
Non-Homogenous Leukoplakia:
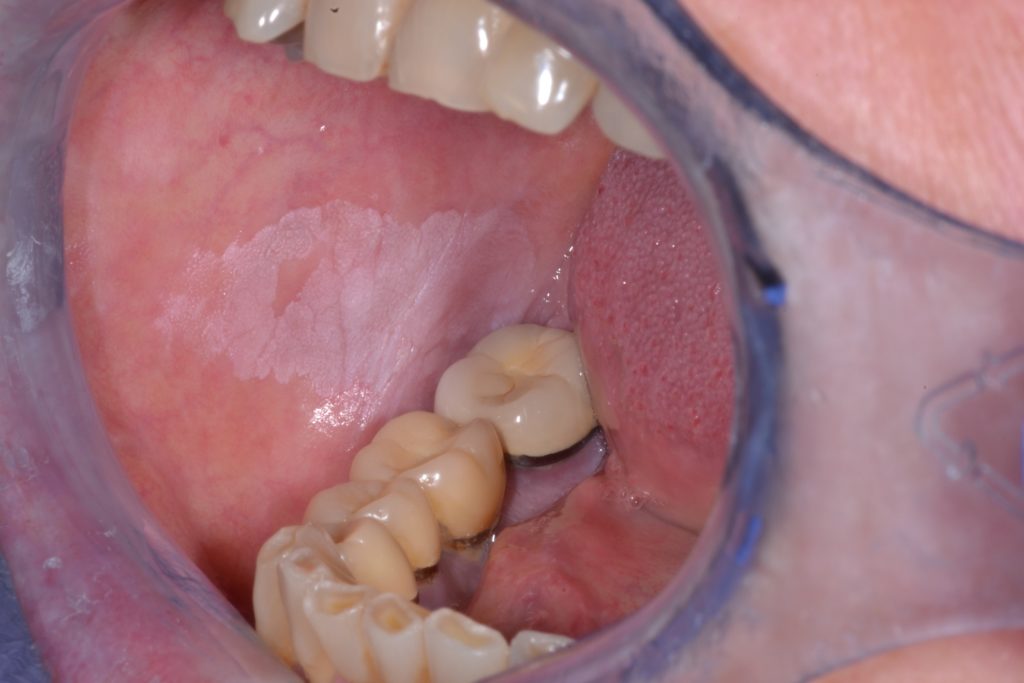
- Characterized by irregular texture, which can include focal superficial ulceration, and ill-defined margins. Non-homogenous leukoplakia may be associated with different clinical appearances used for their description: Further illustrated in Figure 4 & 5.
- Speckled: mixed white and red colour but maintaining predominantly white coloration (also defined as erythroleukoplakia)
- Nodular: small polypoid projections, rounded, red or white excrescences
- Verrucous or exophytic: wrinkled or corrugated surface appearance
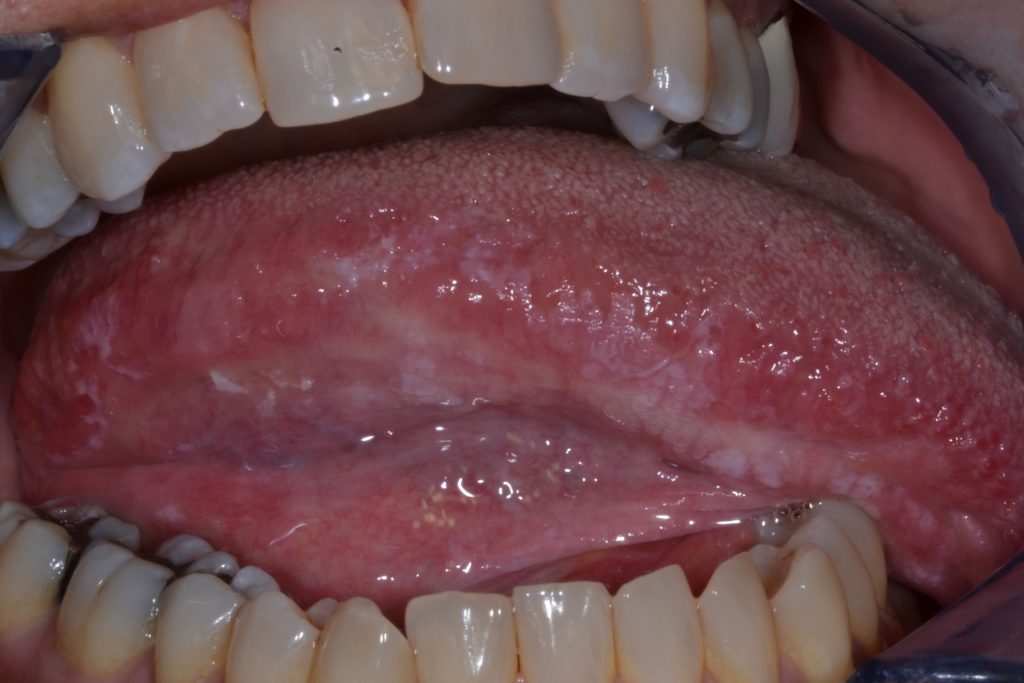
- Leukoplakias are generally asymptomatic and are often identified during routine general examination.
- Homogenous leukoplakias are typically asymptomatic.
- Symptoms are rare, and if present, are usually associated with the non-homogeneous forms which may show focal superficial ulceration.
- Symptoms more frequently reported are discomfort, tingling, and sensitivity to touch, hot beverages, or spicy foods.
Risk of Cancer Onset
Oral Squamous Cell Carcinoma:
- Oral squamous cell carcinoma is the most common neoplasia affecting the oral cavity.
- OSCC typically affects males over 60 years, however, recently prevalence has increased in those younger than 40-years.
- OSCC may present as a non-healing ulcer, growing mass with irregular surface and red or white lesions of the oral epithelium.
Leukoplakia:
- Leukoplakias are classified as oral potentially malignant disorders predisposing affected individuals to an increased risk of development of oral squamous cell carcinoma.
- OL shows variable percentages of progression to OSCC, varying from 0% to 36.4%. The annual rate of transformation ranged from 0.3% to 6.9% per year as reported by a recent review of observational studies.
- Risk factors, which have shown statistical significance, for cancer development in individuals affected by OL are listed in table 4.
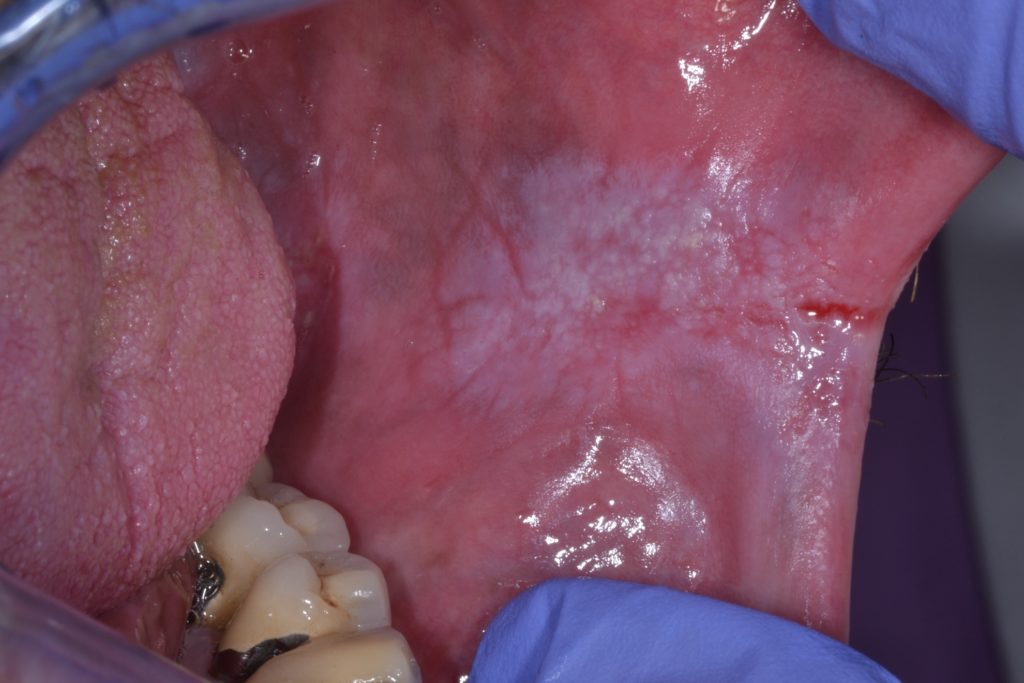
- Figure 6 illustrates a leukoplakia of the floor of mouth, considered a high risk site due to pooling of carcinogens.
| Oral leukoplakia – Risk factor of malignant transformation |
| Non-homogeneous clinical appearance |
| Female gender |
| Long-standing leukoplakia |
| Idiopathic leukoplakia (non-smoking/non-drinking/non- chewing status) |
| Location: lateral tongue and/ or floor of the mouth |
| Size > 200 mm2 |
| Presence of epithelial dysplasia |
| Presence of Candida albicans (chronic hyperplastic candidosis or candida leukoplakia) [35] |
Table 3. Risk factors of malignant transformation for oral leukoplakia
Transformation from Leukoplakia to OSCC:
- Leukoplakias with different clinical, histological or molecular characteristics may have different risks of transforming into OSCC.
- Non-homogenous leukoplakias carry a higher risk of OSCC development compared to homogeneous leukoplakias – there is an approximately 7-fold increase risk for malignant development with non-homogeneous leukoplakia compared with homogeneous leukoplakia and a 5-fold increase in risk when the lesion size is greater than 200 mm2
- In Western populations, elderly females with long-standing idiopathic leukoplakia, with no risk factors, paradoxically, have an increased risk of progression to cancer probably due to an endogenous risk factor rather than environmental factors.
- Redness in an OL lesion (non-homogeneous lesion) indicates potential colonisation by Candida species and also an increased risk for dysplasia and/or malignancy.
- The grade of dysplasia, is accepted a strong predictor of future malignant transformation in OL and remains the best aid to assess the risk of oral cancer development.
- Non- homogeneous leukoplakia more often exhibit severe dysplasia or superficially invasive OSCC at the time of baseline biopsy.
- The risk of cancer development is closely related to the type of lesion and the grade of dysplasia.
Diagnosis of Leukoplakia
A systematic intraoral examination, with palpation of cervical lymph nodes, is mandatory [5]. As described above, the term leukoplakia is a clinical diagnosis having excluded other clinically recognizable white or white/red lesions. The following criteria should be considered when making a clinical diagnosis of oral leukoplakia:
- white patch/plaque that cannot be rubbed off
- homogeneous leukoplakia usually has well-demarcated borders
- non-homogeneous leukoplakia often presents red or nodular components and more diffuse borders
- no evidence of related chronic traumatic irritation
- is not reversible on elimination of apparent traumatic causes
- does not disappear on retracting the tissue
- exclusion of other white or white/red lesions
Carrard and van der Waal recently proposed a list of parameters, some of which are reported in the table that may be relevant when establishing a clinical diagnosis of oral leukoplakia (Table 4).
| Feature | Role and relevance during the clinical diagnosis of oral leukoplakia |
| Age | OL rarely occurs in the first two decades of life. |
| Medical history | Important for the diagnosis of several leukoplakia-like diseases, such as oral manifestations of genodermatoses, syphilis and HIV-infection. |
| Tobacco habits of any type | More common in tobacco users |
| Symptoms | Most OL are asymptomatic, but pain or itching may occur. |
| Onset of the disease | OL arise slowly, over several months or years. |
| Course of the disease | Has a stable clinical course. |
| Morphology | |
| Size | Size is not relevant with regard to a clinical diagnosis of OL. |
| Color | The colour may vary from homogeneous white to a mixed white-and-red appearance. |
| Texture | The texture may vary from smooth, wrinkled to verrucous. Ulceration may be indicative of malignancy. |
| Induration | Palpation is a very important examination tool, induration being a sign of potential malignant nature. |
Table 4: A list of some features, modified from Carrard and van der Waal, which may be useful in the clinical diagnosis and assessment of leukoplakia
- The gold standard procedure to confirm the clinical diagnosis of oral leukoplakia is to perform a representative incisional biopsy of the white patch lesion and send tissue for histopathological analysis.
- Diagnostic biopsy is mandatory to confirm or refute the diagnosis of OL. The biopsy, and the subsequent histopathological examination, allow one to exclude other pathologies which may present as a white patch and assess for candida colonisation within the epithelium.
- Biopsy is necessary to exclude the diagnosis of oral squamous cell carcinoma (OSCC) and to evaluate the presence of and degree of epithelial dysplasia.
- Histopathological findings associated with oral leukoplakia can range from simple epithelial hyperplasia with hyperparakeratosis or hyper(ortho)keratosis, the presence or absence of and degree of epithelial dysplasia.
Though a binary classification for oral dysplasia (low-grade and high-grade) has been proposed, the three-grade system (mild, moderate or severe) is still widely used in clinical practice around the world.
Differential Diagnosis of White Patches
During clinical examination of a white lesion, it is important to evaluate any potential local traumatic causes and, if identified, the white patch should be designated as frictional keratosis. Frictional keratosis is not regarded an OPMD and on removing the putative frictional source, they should resolve.
In the following table is a detailed list of the most relevant white lesions and disorders which should be excluded prior to making a clinical diagnosis of oral leukoplakia (Table 5).
| Differential diagnosis with OL | Description |
| White sponge nevus | Noted in early life, often family history, bilateral presentation, often extensive in the mouth, genital mucosa may be affected. |
| Linea alba | The diagnosis is clinical, almost always bilateral along the plan of occlusion |
| Leukoedema | Clinical diagnosis of a bilateral veil-like aspect on buccal mucosa, and disappears stretching the tissues. Predilection among some racial groups: middle-aged dark-skinned people. |
| Fordyce’s spots/granules | 1-2mm diameter spots/papules distinctly demarcated from the normal surrounding lining mucosa, elevated, circular, white or yellow-white in color |
| Morsicatio buccarum | A condition characterized by chronic irritation or injury to the buccal mucosa, commissures or cheeks, caused by habitual and repetitive chewing or biting. Irregular whitish-yellowish flakes with jagged out line are observed; often bilateral. |
| Frictional keratosis* | Positive clinical history for friction or other mechanical trauma. Mostly reversible after removal of the etiological cause. |
| Keratotic lesions | Include: reversed smoking keratosis, alveolar ridge keratosis (ARK), frictional keratosis, sanguinaria-associated keratosis, tobacco pouch keratosis and keratosis of unknown significance (KUS). Reversed smoking keratosis and tobacco pouch keratosis have malignant potential. |
| Chemical injury | History of prolonged exposure to a chemical agent (e.g., an aspirin tablet) or to a caustic agent (e.g., sodium hypochlorite). The lesion is painful, but usually it resolves rapidly |
| Nicotinic stomatitis (smoker’s palate) | Greyish-white palate with red spots related to inflamed minor salivary glands. Usually, a clinical diagnosis associated with smoking history. |
| Uremic stomatitis | White, sharply demarcated, adherent plaques of fibrinous exudate with some desquamated epithelial cells. Diagnosis is associated with positive history for renal disease. |
| Actinic cheilitis | Inferior lip is the most affected area with white lesion. The typical clinical presentation may vary and leukoplakia-like changes may occur. |
| Oral lichen planus (OLP) | In the typical forms white papules are joined up with lines to form a reticular appearance on the surface of variably inflamed mucosa. Often bilateral presentation. OLP may also present as desquamative gingivitis. Several clinical subtypes of lichen planus may occur: Reticular: lace-like white lines Linear, annular, papular: various presentations as lines, ring-like or white dots Plaque-type: white patch with striae at margins Atrophic, and ulcerative: red and frankly ulcerated Bullous: vesicular OLP plaque-type may be very difficult to distinguish from oral leukoplakia. OLP is included in the group of oral potentially malignant disorders [1] |
| Direct contact lichenoid lesion | Caused by prolonged direct contact of the oral mucosa with an amalgam or other types of restoration. The lesion should disappear in an arbitrarily chosen period of 2-3 months after removal of the restoration. |
| Acute pseudomembranous Candidiasis | Generally widespread infection of oral, and sometimes oropharyngeal mucosa. The white membrane can be scraped off sometimes revealing an erythematous/raw footprint. Associated with local (e.g., inhaled spray steroids) or systemic (e.g., immunodeficiency) underlying causes |
| Chronic hyperplastic candidiasis (Candidal Leukoplakia) | An adherent white or white and red patch caused by a chronic fungal infection, usually Candida albicans. Mainly located in the retro-commissures area, often bilateral. Antifungal treatment is used for evaluate regression of the lesions and establish the diagnosis. |
| Hairy leukoplakia | Keratosis with vertical streaking, caused by opportunistic Epstein Bar virus, which is often bilateral and most common on the lateral borders of the tongue. History of immunosuppression for HIV-disease or drugs (e.g., after organ transplantation/long term steroids). |
| Syphilis, secondary | The diagnosis should evaluate the medical history and demonstrate serology positivity for Treponema Pallidum. The clinical presentation may be various: multiple “mucous patches” whitish lichenoid and leukoplakia-like changes of the oral mucosa multiple red lesions on the dorsal tongue and palate |
| Papilloma and HPV-related lesions (e.g., condyloma acuminatum, multifocal epithelial hyperplasia, verruca vulgaris) | The diagnosis is based on the clinical appearance of the lesions and on the medical history. A biopsy, associated with HPV typing, is usually helpful |
Table 5 A detailed list of the main white lesions, conditions or diseases that should be excluded before making a clinical diagnosis of oral leukoplakia (modified by [1,9])
Management
The primary aim in the management of oral leukoplakia should be to monitor and prevent the oral cancer onset. In a shared attempt to reduce the chances of cancer development, a number of different treatments and management protocols have been proposed. The main approaches in the management of OL can be divided into three groups:
- medical treatment (topical or systemic)
- clinical observation (leave and review); no intervention but strict clinical and histological surveillance)
- surgery: performed with different techniques (scalpel, laser surgery (excision and vaporization, cryosurgery)
There is no consensus on the most appropriate approach for OL.
Medical Treatment
None of the medical and complementary treatments studied (vitamin A, beta carotene, bleomycin) have been shown to be effective in preventing cancer onset in individuals with OL. Several studies on chemo-prevention have also shown considerable toxicity of the drugs used. Medical treatment of leukoplakia may be effective in reducing or resolving oral leukoplakia in the short term, but have been associated with a high risk of relapse. The use of chemo-preventive methods in clinically controlled trials have yet to show their effectiveness in the prevention of malignant transformation and of subsequent recurrence.
Wait and see (Leave and Review) approach
Another approach may be to keep a leukoplakia under strict clinical and histological surveillance, with frequent clinic visits (examination) and biopsies with the aim to detect malignant transformation as early as possible, thus providing the best possible prognosis. This approach is based on the concept of “field cancerization”, proposed by Slaughter in 1957 whereby if a carcinogen has led to a clinically detectable premalignant or malignant change in one part of the oral cavity, there is equal risk to other parts of the oral cavity because of a field effect. OL can be considered an indicator of risk for the whole oral mucosa rather than for a site-specific area. A recent RCT evaluated the effectiveness of surgical excision, compared with standard care in the prevention of OSCC in 260 subjects with non-dysplastic OL. Two patients, one in each arm, developed OSCC and the authors concluded regular clinical follow-up could be considered a reliable standard of care among patients with non-dysplastic OLs.
Surgery
A single Randomized Controlled Trial (RCT) exists, which compared the effect of surgical excision versus no treatment or placebo. Despite this, surgical treatments are, for most clinicians, are the treatment of choice in the management of oral leukoplakia. Surgical excision is frequently performed and there is some evidence to suggest that this reduces the risk of malignant transformation when compared with active surveillance. Only data from observational studies has compared rates of cancer incidence in people who did or did not undergo surgical treatment for oral leukoplakias with differences in diagnostic and inclusion criteria, follow-up intervals, participant characteristics and surgical techniques employed. These studies showed highly variable results and were conflicting in their conclusions .
Surgical intervention with cold knife excision and/ or CO2 laser is usually performed for non-homogeneous leukoplakia or erythroplakia that are associated with moderate to severe dysplasia.
Conclusion
Surgery remains the treatment option favored by most, however the effectiveness of surgery compared with regular clinical observation has not been assessed in RCTs for prevention of cancer development. To date, just one RCT compared surgical treatments to no treatment. In the absence of robust RCTs, clinical practice continues to vary
References and Further Reading
Warnakulasuriya, S.; Kujan, O.; Aguirre‐Urizar, J.M.; Bagan, J. V.; González‐Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2020, odi.13704, doi:10.1111/odi.13704.
van der Waal, I. Oral leukoplakia, the ongoing discussion on definition and terminology. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e685–e692, doi:10.4317/medoral.21007.
Lodi, G.; Franchini, R.; Warnakulasuriya, S.; Varoni, E.M.; Sardella, A.; Kerr, A.R.; Carrassi, A.; Macdonald, L.C.I.; Worthington, H. V. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst. Rev. 2016.
Amagasa, T.; Yamashiro, M.; Uzawa, N. Oral premalignant lesions: From a clinical perspective. Int. J. Clin. Oncol. 2011, 16, 5–14, doi:10.1007/s10147-010-0157-3.
Warnakulasuriya, S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 582–590.
Staines, K.; Rogers, H. Oral leukoplakia and proliferative verrucous leukoplakia: A review for dental
Speight, P.M.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018.
Napier, S.S.; Speight, P.M. Natural history of potentially malignant oral lesions and conditions: An overview of the literature. J. Oral Pathol. Med. 2008, 37, 1–10.
Carrard, V.; van der Waal, I. A Clinical Diagnosis of Oral Leukoplakia . Med Oral Patol Oral Cir Bucal 2018.
Sundberg, J.; Korytowska, M.; Holmberg, E.; Bratel, J.; Wallström, M.; Kjellström, E.; Blomgren, J.; Kovács, A.; Öhman, J.; Sand, L.; et al. Recurrence rates after surgical removal of oral leukoplakia-A prospective longitudinal multicentre study. PLoS One 2019, 14, doi:10.1371/journal.pone.0225682.
Petti, S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003,
Villa, A.; Sonis, S. Oral leukoplakia remains a challenging condition. Oral Dis. 2018, 24, 179–183, doi:10.1111/odi.12781.
Mello, F.W.; Miguel, A.F.P.; Dutra, K.L.; Porporatti, A.L.; Warnakulasuriya, S.; Guerra, E.N.S.; Rivero, E.R.C. Prevalence of oral potentially malignant disorders: A systematic review and
Jayasooriya, P.R.; Dayaratne, K.; Dissanayake, U.B.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: a follow-up study. Clin. Oral Investig. 2020, doi:10.1007/s00784-020-03322-4.
Warnakulasuriya, S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020, 102, doi:10.1016/j.oraloncology.2019.104550.
Arduino, P.; Bagan, J.; El-Naggar, A.; Carrozzo, M. Urban legends series: Oral leukoplakia. Oral Dis. 2013, 19, 642–659.
Villa, A.; Celentano, A.; Glurich, I.; Borgnakke, W.S.; Jensen, S.B.; Peterson, D.E.; Delli, K.; Ojeda, D.; Vissink, A.; Farah, C.S. World Workshop on Oral Medicine VII: Prognostic biomarkers in oral leukoplakia: A systematic review of longitudinal studies. Oral Dis. 2019, 25, 64–78.
Villa, A.; Woo, S. Bin Leukoplakia—A Diagnostic and Management Algorithm. J. Oral Maxillofac. Surg. 2017, 75, 723–734.
Syrjänen, S.; Lodi, G.; von Bültzingslöwen, I.; Aliko, A.; Arduino, P.; Campisi, G.; Challacombe, S.; Ficarra, G.; Flaitz, C.; Zhou, H.; et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011, 17, 58–72, doi:10.1111/j.1601-0825.2011.01792.x.
Varoni, E.M.; Lombardi, N.; Franchini, R.; D’Amore, F.; Noviello, V.; Cassani, B.; Moneghini, L.; Sardella, A.; Lodi, G. Oral Human Papillomavirus (HPV) and sexual behaviors in a young cohort of oral cancer survivors. Oral Dis. 2020, odi.13622, doi:10.1111/odi.13622.
Awadallah, M.; Idle, M.; Patel, K.; Kademani, D. Management update of potentially premalignant oral epithelial lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 628–636.
Warnakulasuriya, S.; Johnson, N.W.; Van Der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580.
van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; present concepts of management. Oral Oncol. 2010, 46, 423–425.
Pentenero, M.; Meleti, M.; Vescovi, P.; Gandolfo, S. Oral proliferative verrucous leucoplakia: Are there particular features for such an ambiguous entity? A systematic review. Br. J. Dermatol. 2014, 170, 1039–1047.
Lombardi, N.; D’amore, F.; Elli, C.; Pispero, A.; Moneghini, L.; Franchini, R. Gingival lesion in proliferative verrucous leukoplakia. Dent. Cadmos 2020, 88, 647–648, doi:10.19256/d.cadmos.10.2020.03.
Hansen, L.S.; Olson, J.A.; Silverman, S. Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surgery, Oral Med. Oral Pathol. 1985, doi:10.1016/0030-4220(85)90313-5.
Villa, A.; Menon, R.S.; Kerr, A.R.; De Abreu Alves, F.; Guollo, A.; Ojeda, D.; Woo, S.B. Proliferative leukoplakia: Proposed new clinical diagnostic criteria. Oral Dis. 2018, 24, 749–760,
Aguirre-Urizar, J.M. Proliferative multifocal leukoplakia better name that proliferative verrucous leukoplakia. World J. Surg. Oncol. 2011, 9.
Lombardi, N.; Varoni, E.M.; Moneghini, L.; Lodi, G. Submucosal oral squamous cell carcinoma of the tongue. Oral Oncol. 2020, 105121, doi:10.1016/j.oraloncology.2020.105121.
Lodi, G.; Tarozzi, M.; Baruzzi, E.; Costa, D.; Franchini, R.; D’Amore, F.; Carassi, A.; Lombardi, N. Odontoiatria e cancro della bocca: dai fattori di rischio alla riabilitazione – Modulo 1: Epidemiologia e fattori di rischio. Dent. Cadmos 2021, 89, 01, doi:10.19256/d.cadmos.01.2021.14.
Lombardi, N.; Sorrentino, D.; D’amore, F.; Moneghini, L.; Franchini, R.; Sardella, A. Oral erythroleukoplakia on the lateral border of the tongue in a young patient. Dent. Cadmos 2020, 88, 277–278, doi:10.19256/d.cadmos.05.2020.03.
Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Pathol. Med. 2016, 45, 155–166, doi:10.1111/jop.12339.
Ho, M.W.; Risk, J.M.; Woolgar, J.A.; Field, E.A.; Field, J.K.; Steele, J.C.; Rajlawat, B.P.; Triantafyllou, A.; Rogers, S.N.; Lowe, D.; et al. The clinical determinants of malignant transformation in oral epithelial dysplasia. Oral Oncol. 2012, 48, 969–976, doi:10.1016/j.oraloncology.2012.04.002.
Field, E.A.; McCarthy, C.E.; Ho, M.W.; Rajlawat, B.P.; Holt, D.; Rogers, S.N.; Triantafyllou, A.; Field, J.K.; Shaw, R.J. The management of oral epithelial dysplasia: The Liverpool algorithm. Oral Oncol. 2015, 51, 883–887.
van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009.
Diz P, Gorsky M, Johnson NW, Kragelund C, Manfredi M, Odell E, et al. Oral leukoplakia and erythroplakia: a protocol for diagnosis and management. EAOM – Diagnostic Ther. Protoc. 2011, 1, 1-8.
Van der Waal, I.; Axéll, T. Oral leukoplakia: A proposal for uniform reporting. Oral Oncol. 2002, 38, 521–526.
Reddi, S.P.; Shafer, A.T. Oral Premalignant Lesions: Management Considerations. Oral Maxillofac. Surg. Clin. North Am. 2006, 18, 425–433.
Pereira, J.D.S.; Carvalho, M.D.V.; Henriques, Á.C.G.; De Queiroz Camara, T.H.; Miguel, M.C.D.C.; Freitas, R.D.A. Epidemiology and correlation of the clinicopathological features in oral epithelial dysplasia: Analysis of 173 cases. Ann. Diagn. Pathol. 2011, 15, 98–102, doi:10.1016/j.anndiagpath.2010.08.008.
Rock, L.D.; Rosin, M.P.; Zhang, L.; Chan, B.; Shariati, B.; Laronde, D.M. Characterization of epithelial oral dysplasia in non-smokers: First steps towards precision medicine. Oral Oncol. 2018, 78, 119–125, doi:10.1016/j.oraloncology.2018.01.028.
Dilhari, A.; Weerasekera, M.M.; Siriwardhana, A.; Maheshika, O.; Gunasekara, C.; Karunathilaka, S.; Nagahawatte, A.; Fernando, N. Candida infection in oral leukoplakia: an unperceived public health problem. Acta Odontol. Scand. 2016, 74, 565–569, doi:10.1080/00016357.2016.1220018.
Speight, P.M. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007, 1, 61–66, doi:10.1007/s12105-007-0014-5.
Warnakulasuriya, S.; Kovacevic, T.; Madden, P.; Coupland, V.H.; Sperandio, M.; Odell, E.; Møller, H. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J. Oral Pathol. Med. 2011, 40, 677–683, doi:10.1111/j.1600-0714.2011.01054.x.
Arduino, P.G.; Surace, A.; Carbone, M.; Elia, A.; Massolini, G.; Gandolfo, S.; Broccoletti, R. Outcome of oral dysplasia: A retrospective hospital-based study of 207 patients with a long follow-up. J. Oral Pathol. Med. 2009, 38, 540–544, doi:10.1111/j.1600-0714.2009.00782.x.
Watabe, Y.; Nomura, T.; Onda, T.; Yakushiji, T.; Yamamoto, N.; Ohata, H.; Takano, N.; Shibahara, T. Malignant transformation of oral leukoplakia with a focus on low-grade dysplasia. J. Oral Maxillofac. Surgery, Med. Pathol. 2016, 28, 26–29, doi:10.1016/j.ajoms.2015.02.007.
Kujan, O.; Oliver, R.J.; Khattab, A.; Roberts, S.A.; Thakker, N.; Sloan, P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006, doi:10.1016/j.oraloncology.2005.12.014.
El-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T., S.P.J. WHO classification of Head and Neck tumors; 4th ed.; IARC Press: Lyon, 2017; ISBN 9789283224372.
Thomson, P. Oral Precancer; Thomson, P., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2012; ISBN 9781118702840.
Warnakulasuriya, S.; Reibel, J.; Bouquot, J.; Dabelsteen, E. Oral epithelial dysplasia classification systems: Predictive value, utility, weaknesses and scope for improvement. J. Oral Pathol. Med. 2008, 37, 127–133.
Pentenero, M.; Carrozzo, M.; Pagano, M.; Galliano, D.; Broccoletti, R.; Scully, C.; Gandolfo, S. Oral mucosal dysplastic lesions and early squamous cell carcinomas: Underdiagnosis from incisional biopsy. Oral Dis. 2003, 9, 68–72, doi:10.1034/j.1601-0825.2003.02875.x.
Lee, J.J.; Hung, H.C.; Cheng, S.J.; Chen, Y.J.; Chiang, C.P.; Liu, B.Y.; Jeng, J.H.; Chang, H.H.; Kuo, Y.S.; Lan, W.H.; et al. Carcinoma and dysplasia in oral leukoplakias in Taiwan: Prevalence and risk factors. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, 472–480, doi:10.1016/j.tripleo.2005.07.024.
Cabay, R.J.; Morton, T.H.; Epstein, J.B. Proliferative verrucous leukoplakia and its progression to oral carcinoma: A review of the literature. J. Oral Pathol. Med. 2007, 36, 255–261, doi:10.1111/j.1600-0714.2007.00506.x.
Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555.
Bagan, J.; Murillo-Cortes, J.; Poveda-Roda, R.; Leopoldo-Rodado, M.; Bagan, L. Second primary tumors in proliferative verrucous leukoplakia: a series of 33 cases. Clin. Oral Investig. 2020, 24, 1963–1969, doi:10.1007/s00784-019-03059-9.
Reibel, J.; Gale, N.; Hille, J.; Hunt, J.; Lingen, M.; Muller, S.; Sloan, P.; Tilakaratne, W.; Westra, W.; Williams, M.; et al. Oral potentially malignant disorders and oral epithelial dysplasia. In WHO classification of head and neck tumours; IARC, Ed.; Lyon France, 2017; pp. 112–115 ISBN 9789283224389.
Ranganathan, K.; Kavitha, L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J. Oral Maxillofac. Pathol. 2019, 23, 19–27.
Barnes, L.; Eveson, J.W.; Reichart, P.; Sidransky, D. World Health Organization Classification of Tumours. Pathology & Genetics. Head and Neck Tumours. International Agency for Research on Cancer (IARC). WHO Classif. Tumours (3rd Ed. 2005, 177–80.
Lee, J.J.; Hung, H.C.; Cheng, S.J.; Chiang, C.P.; Liu, B.Y.; Yu, C.H.; Jeng, J.H.; Chang, H.H.; Kok, S.H. Factors associated with underdiagnosis from incisional biopsy of oral leukoplakic lesions. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 104, 217–225, doi:10.1016/j.tripleo.2007.02.012.
Meleti, M.; Giovannacci, I.; Vescovi, P.; Pedrazzi, G.; Govoni, P.; Magnoni, C. Histopathological determinants of autofluorescence patterns in oral carcinoma. Oral Dis. 2020, doi:10.1111/odi.13304.
Tiwari, L.; Kujan, O.; Farah, C.S. Optical fluorescence imaging in oral cancer and potentially malignant disorders: A systematic review. Oral Dis. 2020.
Warnakulasuriya, S. White, red, and mixed lesions of oral mucosa: A clinicopathologic approach to diagnosis. Periodontol. 2000 2019, 80, 89–104.
Auluck, A.; Pai, K.M. (Mis)interpretations of leukoplakia. J. Can. Dent. Assoc. (Tor). 2005.
Müller, S. Frictional Keratosis, Contact Keratosis and Smokeless Tobacco Keratosis: Features of Reactive White Lesions of the Oral Mucosa. Head Neck Pathol. 2019, 13, 16–24.
Kramer, I.R.; El, L.; Lee, K.W. The clinical features and risk of malignant transformation in sublingual keratosis. Br. Dent. J. 1978, 144, 171–180, doi:10.1038/sj.bdj.4804055.
Eversole, L.R.; Eversole, G.M.; Kopcik, J. Sanguinaria-associated oral leukoplakia: Comparison with other benign and dysplastic leukoplakic lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 89, 455–464, doi:10.1016/S1079-2104(00)70125-9.
Gupta, P.C.; Mehta, F.S.; Daftary, D.K. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10-year follow-up study of Indian villagers. Community Dent. Oral Epidemiol. 1980, 8, 287–333, doi:10.1111/j.1600-0528.1980.tb01302.x.
Sreenivasa Bharath, T.; Govind Raj Kumar, N.; Nagaraja, A.; Saraswathi, T.R.; Suresh Babu, G.; Ramanjaneya Raju, P. Palatal changes of reverse smokers in a rural coastal Andhra population with review of literature. J. Oral Maxillofac. Pathol. 2015, 19, 182–187, doi:10.4103/0973-029X.164530.
Woo, S. Bin; Grammer, R.L.; Lerman, M.A. Keratosis of unknown significance and leukoplakia: A preliminary study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 713–724, doi:10.1016/j.oooo.2014.09.016.
Villa, A.; Hanna, G.J.; Kacew, A.; Frustino, J.; Hammerman, P.S.; Woo, S. Bin Oral keratosis of unknown significance shares genomic overlap with oral dysplasia. Oral Dis. 2019, 25, 1707–1714, doi:10.1111/odi.13155.
Lodi, G.; Porter, S. Management of potentially malignant disorders: Evidence and critique. J. Oral Pathol. Med. 2008, 37, 63–69.
Kanatas, A.N.; Fisher, S.E.; Lowe, D.; Ong, T.K.; Mitchell, D.A.; Rogers, S.N. The configuration of clinics and the use of biopsy and photography in oral premalignancy: A survey of consultants of the British Association of Oral and Maxillofacial Surgeons. Br. J. Oral Maxillofac. Surg. 2011, 49, 99–105, doi:10.1016/j.bjoms.2010.01.016.
Kumar, A.; Cascarini, L.; McCaul, J.A.; Kerawala, C.J.; Coombes, D.; Godden, D.; Brennan, P.A. How should we manage oral leukoplakia? Br. J. Oral Maxillofac. Surg. 2013, 51, 377–383, doi:10.1016/j.bjoms.2012.10.018.
Dionne, K.R.; Warnakulasuriya, S.; Zain, R.B.; Cheong, S.C. Potentially malignant disorders of the oral cavity: Current practice and future directions in the clinic and laboratory. Int. J. Cancer 2015, 136, 503–515.
Diajil, A.; Robinson, C.M.; Sloan, P.; Thomson, P.J. Clinical Outcome Following Oral Potentially Malignant Disorder Treatment: A 100 Patient Cohort Study. Int. J. Dent. 2013, 2013, 1–8, doi:10.1155/2013/809248.
Ribeiro, A.S.; Salles, P.R.; da Silva, T.A.; Mesquita, R.A. A Review of the Nonsurgical Treatment of Oral Leukoplakia. Int. J. Dent. 2010, 2010, 1–10, doi:10.1155/2010/186018.
Jaiswal, G.; Jaiswal, S.; Kumar, R.; Sharma, A. Field cancerization: concept and clinical implications in head and neck squamous cell carcinoma. J. Exp. Ther. Oncol. 2013.
Arduino, P.G.; Lodi, G.; Cabras, M.; Macciotta, A.; Gambino, A.; Conrotto, D.; Karimi, D.; El Haddad, G.; Carbone, M.; Broccoletti, R. A Randomized Controlled Trial on Efficacy of Surgical Excision of non-dysplastic Leukoplakia to Prevent Oral Cancer. Cancer Prev. Res. 2020, canprevres.0234.2020, doi:10.1158/1940-6207.capr-20-0234.
Mehanna, H.M.; Rattay, T.; Smith, J.; McConkey, C.C. Treatment and follow-up of oral dysplasia – A systematic review and meta-analysis. Head Neck 2009, doi:10.1002/hed.21131.
Saito, T.; Sugiura, C.; Hirai, A.; Notani, K.I.; Totsuka, Y.; Shindoh, M.; Fukuda, H. Development of squamous cell carcinoma from pre-existent oral leukoplakia: With respect to treatment modality. Int. J. Oral Maxillofac. Surg. 2001, 30, 49–53.
Zhang, L.; Lubpairee, T.; Laronde, D.M.; Rosin, M.P. Should severe epithelial dysplasia be treated? Oral Oncol. 2016, 60, 125–129, doi:10.1016/j.oraloncology.2016.07.007.
Balasundaram, I.; Payne, K.F.B.; Al-Hadad, I.; Alibhai, M.; Thomas, S.; Bhandari, R. Is there any benefit in surgery for potentially malignant disorders of the oral cavity? J. Oral Pathol. Med. 2014.
Thomson, P.J.; McCaul, J.A.; Ridout, F.; Hutchison, I.L. To treat…or not to treat? Clinicians’ views on the management of oral potentially malignant disorders. Br. J. Oral Maxillofac. Surg. 2015, 53, 1027–1031, doi:10.1016/j.bjoms.2015.08.263.
Kuribayashi, Y.; Tsushima, F.; Morita, K.; Matsumoto, K.; Sakurai, J.; Uesugi, A.; Sato, K.; Oda, S.; Sakamoto, K.; Harada, H. Long-term outcome of non-surgical treatment in patients with oral leukoplakia. Oral Oncol. 2015, 51, 1020–1025, doi:10.1016/j.oraloncology.2015.09.004.
Mogedas-Vegara, A.; Hueto-Madrid, J.A.; Chimenos-Küstner, E.; Bescós-Atín, C. Oral leukoplakia treatment with the carbon dioxide laser: A systematic review of the literature. J. Cranio-Maxillofacial Surg. 2016, doi:10.1016/j.jcms.2016.01.026.







