DEFINITION
PVL can be considered a distinct form of oral leukoplakia (OL) characterized by multifocal lesions, progressive clinical course and changes in clinical appearance and histopathologic features. However, such a position to classify PVL as a separate entity is not universally agreed, since large, widespread leukoplakias, and not only PVLs, carry a distinct risk of malignant transformation.
In 2020, the Working Group of the WHO Collaborating Centre for Oral Cancer acknowledged that “despite the imperfection of the term PVL to capture an expanded group of patients with multifocal disease, the term is widely reported, and the Working Group recommended retaining this term”. The same group proposed the following definition: “a progressive, persistent, and irreversible disorder characterized by the presence of multiple leukoplakias that frequently become warty”.
Epidemiology and Risk of Cancer
- The overall prevalence of OL is estimated to range between 1.5% and 2.6%. PVL is less common than standard leukoplakia and data on its prevalence is not available.
- The aetiology of PVL remains unknown.
- PVL is more common in elderly females without a racial predilection and it is not associated with the traditional risk factors for OL and oral squamous cell carcinoma (OSCC), i.e. tobacco and alcohol consumption.
- Approximately two-thirds of PVL cases are in never-smokers and patients who do not report alcohol abuse.
- The reported HPV prevalence in PVL ranges from 0-88% based on the results from studies with small patient numbers.
- A study investigating the prevalence of HPV in 58 patients showed no significant differences between patients affected by PVL and those with conventional OL (24% versus 25%)
- On the first histopathological examination, the majority of PVL lesions (52.5%) show hyperkeratosis without dysplasia or verrucous hyperplasia.
- Nevertheless, PVL is associated with the highest incidence of oral cancer amongst OPMDs, either verrucous carcinoma or squamous cell carcinoma.
- Approximately 61% of patients with PVL develop oral cancer over an average period of 7.4 years, though a systematic review estimated malignant transformation occurs in 49.5% (CI 26.7%-72.4%) of PVL cases (Spriano et al., 2020) .
- The annual incidence is estimated to be 10.0% per year.
- Multiple primary carcinomas of gingival sites have been reported in the literature in patients affected by PVL, evidenced in recent case series.
- PVL is associated with an overall mortality of 40%.
Clinical Presentation
Clinically, PVL may involve a single large area of the oral mucosa, but more frequently, it is multifocal, affecting the gingiva, buccal mucosa, and tongue in both contiguous and non-contiguous sites of the oral cavity. The oral sites more frequently involved by PVL are the gingiva (62.7%), the buccal mucosa (59.8%) and the tongue (49.1%). Figures 1-4 illustrate PVL affecting multiple sites within the oral cavity.
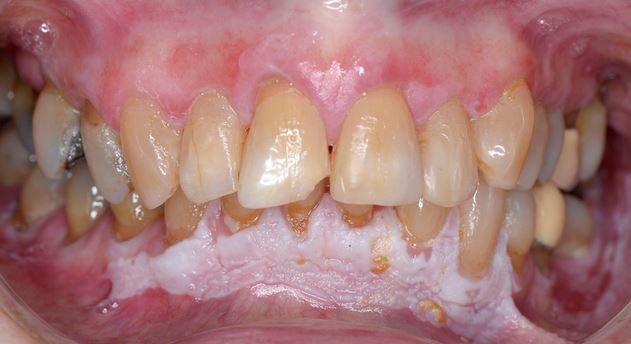
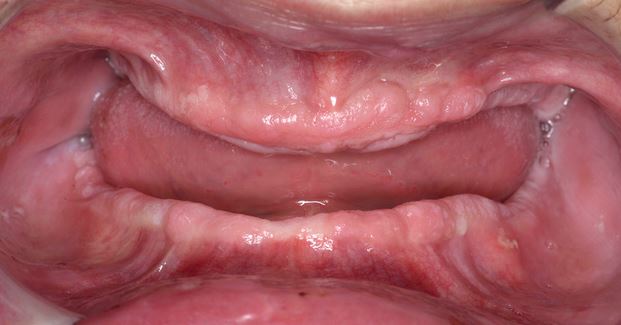
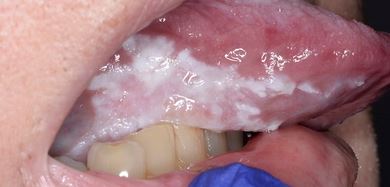
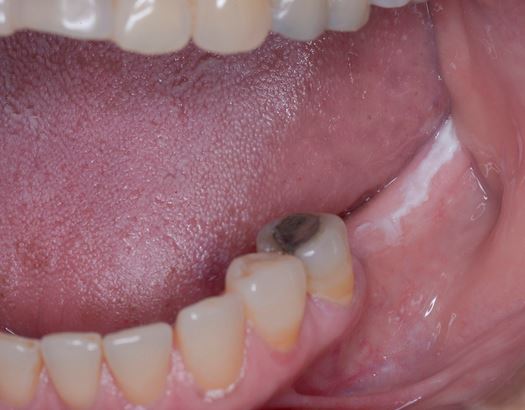
Multifocality and progressive clinical course are the main features which characterize PVL. The oral lesions in PVL are often associated with ongoing changes in the clinical and histopathological picture. Table 1 reports the characteristic clinical features of PVL as described in the recent report on OPMDs prepared by the WHO Collaborating Centre for Oral Cancer.
| PVL clinical presentation |
| Multiple, thick, white patches in more than two different oral sites, frequently found on the gingiva, alveolar processes, and palate |
| Majority of the lesion presents a verrucous pattern |
| Lesions spread and coalesce during development |
| Recurrence in a previously treated area |
Table 1: Clinical presentation of PVL according with WHO Collaborating Centre for Oral Cancer 2020 [4].
PVL usually begins as a homogenous leukoplakia with no dysplasia on histological examination and progressively evolves to verrucous lesions in single or multiple area of the oral mucosa. At the beginning, PVL manifests as one or more leukoplakias which progressively affect multiple locations with gradual spread of a single lesion or multiple lesions coalescing [3,4]. At the initial stages, PVL may present as a multifocal lesion lacking the typical verrucous appearance. In addition, the initial lesion can be white and flat, sometimes demonstrating a lichenoid appearance, making the distinction between it and oral lichen planus (OLP) difficult [3,4,28,29]. In such cases, PVL may be erroneously treated as OLP for long periods with the subsequent risk of missing or delaying the diagnosis of a potential OSCC.
PVL has also been characterized by prominent erythema (19% in the series by Villa et al) with a malignant transformation rate (MTR) of 100% when compared to those PVL lesions without an erythematous component (MTR 62.5%).
Diagnosis
- The histopathological features of PVL are not pathognomonic and are non-specific, ranging from hyperkeratosis in the early stages, to verrucous hyperplasia, and different grades of dysplasia.
- In addition, PVL cases characterized clinically by an inflammatory component or lichenoid appearance may show a “lichenoid” lymphocytic band, and be misdiagnosed as OLP.
- For this reason, the definitive diagnosis of PVL is made on the basis of a combination of clinical and histopathological findings.
- Every white lesion, which becomes warty and exophytic, with spread over time and recurrence after treatment, should be considered as PVL.
Since the first definition in 1985, other authors have proposed alternative diagnostic criteria. In 2010, Cerero-Lapiedra et al proposed five major and four minor criteria, and specific combinations of them, to establish an early diagnosis of PVL (Tab.2).
| Major Criteria | Minor Criteria |
| Oral leukoplakia affecting more than two different oral sites (more frequently gingiva, palate, and alveolar ridge) | The lesions cover a mucosal extension that exceeds 3 cm (adding all the affected areas) |
| Verrucous clinical appearance | Female gender |
| Lesions show clinical progression and spreading | Never-smoker (regardless of gender) |
| Recurrence in a previously treated area | Disease evolution longer than 5 years |
| Histopathological picture of oral epithelial hyperkeratosis or verrucous hyperplasia, verrucous carcinoma, or squamous cell carcinoma. |
Diagnosis of PVL can be made when:
three major criteria (histopathological criterion being one)
or two major (histopathological criterion being one) and two minor criteria
Table 2: PVL diagnostic criteria according to Cicero-Lapiedra et al. (Modified by [3,20]).
Carrard et al proposed to simplify the diagnostic criteria of PVL omitting the distinction between major and minor criteria, and suggested four criteria which should be met (Table 3) .
| Modified diagnostic criteria for PVL: all four criteria should be met. |
| Leukoplakia showing the presence of verrucous or wartlike areas, involving more than two oral subsites. The following oral subsites are recognized: dorsum of the tongue (unilateral or bilateral), border of the tongue, cheek mucosa, alveolar mucosa or gingiva upper jaw, alveolar mucosa or gingiva lower jaw, hard and soft palate, floor of the mouth, upper lip and lower lip. |
| Adding all involved sites, the minimum seize should be at least three centimeters. |
| A well-documented period of disease evolution of at least five years, being characterized by spreading and enlarging and the occurrence of one or more recurrences in a previously treated area |
| The availability of at least one biopsy in order to rule out the presence of a verrucous carcinoma or squamous cell carcinoma |
Table 3: Simplified diagnostic criteria for PVL.
- When obtaining a biopsy from a suspected PVL lesion, as well as non-homogeneous OL, it is important to choose the most representative parts to avoid underdiagnosis.
- Histopathological examination may lead to a variable diagnosis within the same lesion. For this reason, multiple mapping biopsies are often recommended. Multiple and periodic mapping biopsies are indicated in PVL to detect different grades of dysplasia or to exclude OSCC.
- It has been reported in PVL and non-homogeneous OL that approximately 10%–17% of OSCC cases may be missed by performing only a single biopsy.
Management
- The management and treatment of PVL is particularly challenging. In the last decade, different approaches have been proposed to reduce the high incidence of oral cancer among patients affected by PVL.
- To date, none of the treatments, (surgery, laser ablation, retinoids, photodynamic therapy and chemotherapy) have proven to be effective in reducing oral cancer development in these patients .
- Despite the lack of evidence on efficacy, surgical treatment is the most frequent intervention adopted in the management of PVL .
- When PVL is characterized by small lesions, and if the area is discrete, excision may be attempted, but the patient should be aware that ongoing follow-up will be necessary due to high oral cancer risk and risk of lesion recurrence (71%).
- Most PVL cases demonstrate large lesions and involve multifocal non-contiguous areas.
- A recent systematic review reported the overall recurrence rate of PVL to all treatment modalities is approximately 85%, without any reduction in cancer incidence.
- For all these reasons, surgical excision in extensive PVL may be considered an impractical and unnecessary aggressive treatment option.
- Many clinicians choose a “wait and see” approach, based on periodical multiple biopsies and regular clinical examination.
- The aim of this approach is to identify very early onset of oral carcinoma, giving the best treatment outcome and prognosis.
In conclusion, considering the high cancer incidence in PVL patients, multiple biopsies and strict clinical follow-up is mandatory (every 3-6 months depending on clinical features). The timing of malignant transformation is unpredictable, therefore lifelong follow-up is necessary.
References and Further Reading
1. Hansen, L.S.; Olson, J.A.; Silverman, S. Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surgery, Oral Med. Oral Pathol. 1985, 60, 285–298, doi:10.1016/0030-4220(85)90313-5.
2. Aguirre-Urizar, J.M. Proliferative multifocal leukoplakia better name that proliferative verrucous leukoplakia. World J. Surg. Oncol. 2011, 9, 122, doi:10.1186/1477-7819-9-122.
3. Villa, A.; Menon, R.S.; Kerr, A.R.; De Abreu Alves, F.; Guollo, A.; Ojeda, D.; Woo, S.B. Proliferative leukoplakia: Proposed new clinical diagnostic criteria. Oral Dis. 2018, 24, 749–760, doi:10.1111/odi.12830.
4. Warnakulasuriya, S.; Kujan, O.; Aguirre‐Urizar, J.M.; Bagan, J. V.; González‐Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2020, odi.13704, doi:10.1111/odi.13704.
5. Villa, A.; Sonis, S. Oral leukoplakia remains a challenging condition. Oral Dis. 2018, 24, 179–183, doi:10.1111/odi.12781.
6. van der Waal, I. Oral leukoplakia; a proposal for simplification and consistency of the clinical classification and terminology. Med. Oral Patol. Oral y Cir. Bucal 2019, 24, e799–e803, doi:10.4317/medoral.23372.
7. Petti, S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003, 39, 770–780, doi:10.1016/S1368-8375(03)00102-7.
8. Lombardi, N.; D’amore, F.; Elli, C.; Pispero, A.; Moneghini, L.; Franchini, R. Gingival lesion in proliferative verrucous leukoplakia. Dent. Cadmos 2020, 88, 647–648, doi:10.19256/d.cadmos.10.2020.03.
9. Staines, K.; Rogers, H. Oral leukoplakia and proliferative verrucous leukoplakia: A review for dental practitioners. Br. Dent. J. 2017, 223, 655–661, doi:10.1038/sj.bdj.2017.881.
10. Varoni, E.M.; Lombardi, N.; Franchini, R.; D’Amore, F.; Noviello, V.; Cassani, B.; Moneghini, L.; Sardella, A.; Lodi, G. Oral Human Papillomavirus (HPV) and sexual behaviors in a young cohort of oral cancer survivors. Oral Dis. 2021, 27, 919–923, doi:10.1111/odi.13622.
11. Bagan, J. V.; Jimenez, Y.; Murillo, J.; Gavaldá, C.; Poveda, R.; Scully, C.; Alberola, T.M.; Torres-Puente, M.; Pérez-Alonso, M. Lack of Association Between Proliferative Verrucous Leukoplakia and Human Papillomavirus Infection. J. Oral Maxillofac. Surg. 2007, 65, 46–49, doi:10.1016/j.joms.2005.12.066.
12. Palefsky, J.M.; Silverman, S.; Abdel‐Salaam, M.; Daniels, T.E.; Greenspan, J.S. Association between proliferative verrucous leukoplakia and infection with human papillomavirus type 16. J. Oral Pathol. Med. 1995, 24, 193–197, doi:10.1111/j.1600-0714.1995.tb01165.x.
13. Campisi, G.; Giovannelli, L.; Ammatuna, P.; Capra, G.; Colella, G.; Di Liberto, C.; Gandolfo, S.; Pentenero, M.; Carrozzo, M.; Serpico, R.; et al. Proliferative verrucous vs conventional leukoplakia: No significantly increased risk of HPV infection. Oral Oncol. 2004, 40, 835–840, doi:10.1016/j.oraloncology.2004.02.007.
14. Cabay, R.J.; Morton, T.H.; Epstein, J.B. Proliferative verrucous leukoplakia and its progression to oral carcinoma: A review of the literature. J. Oral Pathol. Med. 2007, 36, 255–261, doi:10.1111/j.1600-0714.2007.00506.x.
15. Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555.
16. Villa, A.; Celentano, A.; Glurich, I.; Borgnakke, W.S.; Jensen, S.B.; Peterson, D.E.; Delli, K.; Ojeda, D.; Vissink, A.; Farah, C.S. World Workshop on Oral Medicine VII: Prognostic biomarkers in oral leukoplakia: A systematic review of longitudinal studies. Oral Dis. 2019, 25, 64–78.
17. Lodi, G.; Tarozzi, M.; Baruzzi, E.; Costa, D.; Franchini, R.; D’Amore, F.; Carassi, A.; Lombardi, N. Odontoiatria e cancro della bocca: dai fattori di rischio alla riabilitazione – Modulo 1: Epidemiologia e fattori di rischio. Dent. Cadmos 2021, 89, 01, doi:10.19256/d.cadmos.01.2021.14.
18. Lombardi, N.; Varoni, E.M.; Moneghini, L.; Lodi, G. Submucosal oral squamous cell carcinoma of the tongue. Oral Oncol. 2021, 115, 105121, doi:10.1016/j.oraloncology.2020.105121.
19. Capella, D.L.; Gonçalves, J.M.; Abrantes, A.A.A.; Grando, L.J.; Daniel, F.I. Proliferative verrucous leukoplakia: diagnosis, management and current advances. Braz. J. Otorhinolaryngol. 2017, 83, 585–593, doi:10.1016/j.bjorl.2016.12.005.
20. Cerero-Lapiedra, R.; Balade-Martinez, D.; Moreno-Lopez, L.; Esparza-Gomez, G.; Bagan, J. Proliferative verrucous leukoplakia: A proposal for diagnostic criteria. Med. Oral Patol. Oral y Cir. Bucal 2010, 15, e839–e845, doi:10.4317/medoral.15.e839.
21. Pentenero, M.; Meleti, M.; Vescovi, P.; Gandolfo, S. Oral proliferative verrucous leucoplakia: Are there particular features for such an ambiguous entity? A systematic review. Br. J. Dermatol. 2014, 170, 1039–1047.
22. Villa, A.; Woo, S. Bin Leukoplakia—A Diagnostic and Management Algorithm. J. Oral Maxillofac. Surg. 2017, 75, 723–734.
23. Bagan, J.; Murillo-Cortes, J.; Poveda-Roda, R.; Leopoldo-Rodado, M.; Bagan, L. Second primary tumors in proliferative verrucous leukoplakia: a series of 33 cases. Clin. Oral Investig. 2020, 24, 1963–1969, doi:10.1007/s00784-019-03059-9.
24. Abadie, W.M.; Partington, E.J.; Fowler, C.B.; Schmalbach, C.E. Optimal Management of Proliferative Verrucous Leukoplakia: A Systematic Review of the Literature. Otolaryngol. – Head Neck Surg. (United States) 2015, 153, 504–511, doi:10.1177/0194599815586779.
25. Warnakulasuriya, S.; Johnson, N.W.; Van Der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580.
26. Ghazali, N.; Bakri, M.M.; Zain, R.B. Aggressive, multifocal oral verrucous leukoplakia: proliferative verrucous leukoplakia or not? J. Oral Pathol. Med. 2003, 32, 383–392, doi:10.1034/j.1600-0714.2003.00180.x.
27. Warnakulasuriya, S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 582–590.
28. Garcia-Pola, M.; Llorente-Pendas, S.; Gonzalez-Garcia, M.; Garcia-Martin, J. The development of proliferative verrucous leukoplakia in oral lichen planus. A preliminary study. Med. Oral Patol. Oral y Cir. Bucal 2016, 21, e328–e334, doi:10.4317/medoral.20832.
29. McParland, H.; Warnakulasuriya, S. Lichenoid morphology could be an early feature of oral proliferative verrucous leukoplakia. J. Oral Pathol. Med. 2021, 50, 229–235, doi:10.1111/jop.13129.
30. Carrard, V.C.; Brouns, E.R.E.A.; van der Waal, I. Proliferative verrucous leukoplakia; A critical appraisal of the diagnostic criteria. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e411–e413, doi:10.4317/medoral.18912.
31. Warnakulasuriya, S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020, 102, 104550, doi:10.1016/j.oraloncology.2019.104550.
32. Pentenero, M.; Carrozzo, M.; Pagano, M.; Galliano, D.; Broccoletti, R.; Scully, C.; Gandolfo, S. Oral mucosal dysplastic lesions and early squamous cell carcinomas: Underdiagnosis from incisional biopsy. Oral Dis. 2003, 9, 68–72, doi:10.1034/j.1601-0825.2003.02875.x.
33. Lee, J.J.; Hung, H.C.; Cheng, S.J.; Chiang, C.P.; Liu, B.Y.; Yu, C.H.; Jeng, J.H.; Chang, H.H.; Kok, S.H. Factors associated with underdiagnosis from incisional biopsy of oral leukoplakic lesions. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 104, 217–225, doi:10.1016/j.tripleo.2007.02.012.
34. Lodi, G.; Franchini, R.; Warnakulasuriya, S.; Varoni, E.M.; Sardella, A.; Kerr, A.R.; Carrassi, A.; MacDonald, L.C.; Worthington, H. V.; Mauleffinch, L.F. Limited evidence for interventions to treat oral leukoplakia. Evid. Based. Dent. 2017, 18, 92–93.
35. Thomson, P.J.; McCaul, J.A.; Ridout, F.; Hutchison, I.L. To treat…or not to treat? Clinicians’ views on the management of oral potentially malignant disorders. Br. J. Oral Maxillofac. Surg. 2015, 53, 1027–1031, doi:10.1016/j.bjoms.2015.08.263.







