The World Health Organisation (WHO) originally defined erythroplakia as ‘any lesion of the oral mucosa that presents as bright red velvety plaques which cannot be characterised clinically or pathologically as any other recognizable condition’. There is no universally accepted definition of oral erythroplakia, however, the majority of modern definitions, are more or less reductive of the original WHO definition. The most frequently used definition in the literature described as oral erythroplakia as a ‘a fiery red patch that cannot be characterized clinically or pathologically as any other definable lesion’
Additional terminology such as erythroleukoplakia, leukoerythroplakia and speckled erythroplakia, have been used to describe a mixture of red and white patches.
Aetiopathogenesis
- Many of the main aetiological factors in oral cancer are contributory or aetiological factors in the development of erythroplakia.
- It has been observed that these factors are contributory to the malignant transformation of erythroplakia, however, their role in the aetiopathogenesis of erythroplakia is poorly understood.
- These factors are primarily betel quid chewing, tobacco chewing, tobacco smoking and alcohol consumption. Other implicated possible aetiological factors include BMI and nutritional status
- Human papillomavirus (HPV) has also been elucidated as a possible contributory factor or aetiological cofactor.
Why are they red?
- Histopathological analysis reveals dysplasia (varying from mild to severe), carcinoma in situ or squamous cell carcinoma in 60-90% of cases. These microscopic descriptions would explain the deep red colour of the lesions.
- There is lack of surface keratin which would normally disperse and reduce the intensity of the red colour
- There is also a reduction in the depth of the epithelial layer; therefore, vasculature present in the connective tissue are more visible from the surface.
- Finally, the number of vascular structures appears to increase in response to the inflammation
Epidemiology
- The prevalence rate varies from approximately 0.02 to 2%.
- It is widely accepted that the prevalence of erythroplakia is less than other OPMDs and oral leukoplakia.
- There appears to be no gender predilection, despite earlier studies reporting a male predominance.
- Erythroplakia appears to occur mainly in middle aged and elderly (Reichart and Philipsen, 2005).
- There is no known geographical distribution of erythroplakia, however, the prevalence is observably higher in populations with a preexisting smoking and betel nut chewing habit.
Clinical Presentation
- Presents as fiery red macule or patch with a soft velvety texture.
- Typically soft to palpate with induration only being observed in cases of malignancy.
- Lesions are usually irregular in outline, though, well defined. Occasionally, however, the surface may appear granular.
- There are usually no symptoms. Patients may report non-specific soreness or burning from the area. Metallic taste have also been reported.
ERYTHROLEUKOPLAKIA
- Red areas show atrophy or thinning of epithelium and unlike erythroplakia or leukoplakia, may present with an irregular margin.
- Erythroleukoplakia is more commonly symptomatic.
- The floor of the mouth, ventral tongue, soft palate, tonsillar fauces and the buccal mucosa are the sites reported to be the most commonly affected.
- Typically, lesions are small (less than 1.5cm) though larger lesions have been reported (>4cm).
- Rarely erythroplakia affects multiple sites.
- In cases of malignant transformation, patients may present with signs and symptoms of oral squamous cell carcinoma (OSCC).
- There is no widely accepted classification of erythroplakia.
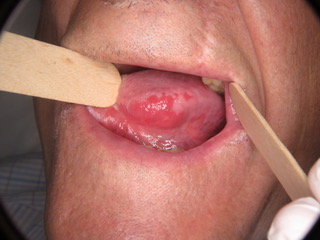
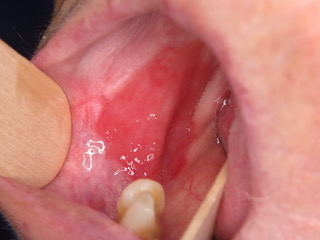
Figure 1: Widespread Erythroplakia homogenously affecting the left lateral border of the tongue and homogenous erythroplakia homogenously affecting the right buccal mucosa posteriorly.
Differential Diagnosis
The list of differentials includes those lesions presenting with erythematous oral mucosa. Erythematous candidiasis and atrophic lichen planus are frequently cited as the most common.
Erythematous candidiasis and denture stomatitis can be differentiated from erythroplakia, as frequently these erythematous areas resolve with denture/oral hygiene instruction and treatment with antifungals, both systemic and topicals agents.
Erythematous candidiasis may present asymptomatically, with central erythematous areas affecting the dorsal surface of the tongue, with a reflective lesion affecting the palate.
Other microbial infections may present with oral manifestations such as red patches affecting the oral mucosa, including histoplasmosis and tuberculosis.
Atrophic lichen planus tends to present symptomatically, with other features of OLP such as white striations affecting other areas of the oral mucosa. Oral manifestations of systemic lupus erythematous (SLE) are frequently encountered and may include non-specific erythema.
Diagnosis
- A solitary lesion with well demarcated borders aids the practitioner to clinically distinguish erythroplakia from other disorders.
- These solitary lesions may be discovered incidentally by the general dental practitioners prompting referral.
- Urgent biopsy is essential to exclude neoplastic change.
- The histopathological features that may be found have been described above, thin and atrophic epithelium, lack of keratin and hyperplasia. Hematoxylin and eosin is the most common staining used by histopathologists in the diagnosis of erythroplakia.
Malignant Transformation:
- The rate of malignant transformation varies from 14 to 50%.
- Many red lesions may present with carcinoma in situ or invasive disease at the time of diagnosis.
- The presence of moderate to severe dysplasia indicates a considerably higher risk of malignant transformation.
- A recent systematic review calculated the overall risk of malignant transformation as 33.1% and a malignant transformation rate per year at 2.7%.
Management
Owing to the highest risk of malignant transformation early treatment is crucial.
- Surgical excision is the treatment of choice.
- There is lack of studies on follow-up following surgical excision and treatment outcomes.
- There are no guidelines on the exact margin to excise, this is often dictated by the degree of cellular atypia identified.
- Carbon dioxide laser excision has been used recently, as an alternative to scalpel excision. This has been associated with more favourable healing of the area and post-operative comfort.
- The rate of recurrence following excision is unknown, however, the size of the excised lesion has been shown to be a significant predictive factor.
- Other therapies including vitamin A, retinoids, bleomycin and mixed tea have not yielded favourable results to advocate their use.
- Cessation of recreational habits associated with higher risk of malignant transformation should be recommended.
Follow-up:
- There is no suggested follow-up interval for erythroplakia.
- An increase in size, high risk recreational habits (smoking/alcohol), high risk sites (ventral tongue and floor of mouth), change in clinical appearance and the development of symptoms may dictate re-biopsy of a lesion.
- A clinical photograph taken at subsequent appointments is useful to objectively assess for any changes in appearance of the lesion.
Summary
The most widely accepted definition of erythroplakia is ‘any lesion of the oral mucosa that presents as bright red velvety plaques which cannot be characterized clinically or pathologically as any other recognizable condition’. Smoking, alcohol, betel nut chewing and genetic mutations may all play role in aetiology. Erythroplakia is the OPMD with the lowest prevalence rate and the highest malignant transformation rate. Considering the high malignant transformation rate, early incisional biopsy is advocated to identify early neoplastic changes. The treatment of choice for high-risk lesions (severe dysplasia and carcinoma in situ) is surgical excision. Regular follow is necessary and the threshold to for re-biopsy is low.
Despite the highest malignant transformation rate amongst OPMDs, erythroplakia remains the OPMD that is most poorly understood. The lack of research leaves many unanswered questions regarding aetiology, prevalence, diagnosis and management of erythroplakia.
References and Further Reading
J.J. Pindborg, P.A. Reichart, C.J. Smith, I. van der Waal, in collaboration with Sobin LH and pathologists in 9 countries. Histological typing of cancer and precancer of the oral mucosa
(2nd ed.), Springer Verlag, Berlin, New York, Tokyo (1997)
C. Scully Oral and maxillofacial medicine the basis of diagnosis and treatment, Wright. Elsevier Science Ltd., Edinburgh, London, New York (2004), pp. 289-290
J. PHILIP SAPP DDS, MS, … GEORGE P. WYSOCKI DDS, PHD, in Contemporary Oral and Maxillofacial Pathology (Second Edition), 2004
Abati, S., Bramati, C., Bondi, S., Lissoni, A. and Trimarchi, M. (2020) ‘Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis’, Int J Environ Res Public Health, 17(24).
Al-Attas, S. A., Ibrahim, S. S., Amer, H. A., Darwish, Z. l.-S. and Hassan, M. H. (2014) ‘Prevalence of potentially malignant oral mucosal lesions among tobacco users in Jeddah, Saudi Arabia’, Asian Pac J Cancer Prev, 15(2), pp. 757-62.
Bouquot, J. E. and Ephros, H. (1995) ‘Erythroplakia: the dangerous red mucosa’, Pract Periodontics Aesthet Dent, 7(6), pp. 59-67; quiz 68.
Fatahzadeh, M. and Schwartz, R. A. (2013) ‘Oral Kaposi’s sarcoma: a review and update’, Int J Dermatol, 52(6), pp. 666-72.
Ferreira, A. M., de Souza Lucena, E. E., de Oliveira, T. C., da Silveira, É., de Oliveira, P. T. and de Lima, K. C. (2016) ‘Prevalence and factors associated with oral potentially malignant disorders in Brazil’s rural workers’, Oral Dis, 22(6), pp. 536-42.
Hashibe, M., Mathew, B., Kuruvilla, B., Thomas, G., Sankaranarayanan, R., Parkin, D. M. and Zhang, Z. F. (2000) ‘Chewing tobacco, alcohol, and the risk of erythroplakia’, Cancer Epidemiol Biomarkers Prev, 9(7), pp. 639-45.
Holmstrup, P. (2018) ‘Oral erythroplakia-What is it?’, Oral Dis, 24(1-2), pp. 138-143.
Holmstrup, P. and Pindborg, J. J. (1979) ‘Erythroplakic lesions in relation to oral lichen planus’, Acta Derm Venereol Suppl (Stockh), 59(85), pp. 77-84.
Iocca, O., Sollecito, T. P., Alawi, F., Weinstein, G. S., Newman, J. G., De Virgilio, A., Di Maio, P., Spriano, G., Pardiñas López, S. and Shanti, R. M. (2020) ‘Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype’, Head Neck, 42(3), pp. 539-555.
Kim, D. H., Lee, J., Lee, M. H., Kim, S. W. and Hwang, S. H. (2020) ‘Efficacy of chemiluminescence in the diagnosis and screening of oral cancer and precancer: a systematic review and meta-analysis’, Braz J Otorhinolaryngol.
Kramer, I. R., Lucas, R. B., Pindborg, J. J. and Sobin, L. H. (1978) ‘Definition of leukoplakia and related lesions: an aid to studies on oral precancer’, Oral Surg Oral Med Oral Pathol, 46(4), pp. 518-39.
Lumerman, H., Freedman, P. and Kerpel, S. (1995) ‘Oral epithelial dysplasia and the development of invasive squamous cell carcinoma’, Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 79(3), pp. 321-9.
Mello, F. W., Miguel, A. F. P., Dutra, K. L., Porporatti, A. L., Warnakulasuriya, S., Guerra, E. N. S. and Rivero, E. R. C. (2018) ‘Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis’, J Oral Pathol Med, 47(7), pp. 633-640.
MERRICKS, J. W. and COTTRELL, T. L. (1953) ‘Erythroplasia of Queyrat’, J Urol, 69(6), pp. 807-13.
Nielsen, H., Norrild, B., Vedtofte, P., Praetorius, F., Reibel, J. and Holmstrup, P. (1996) ‘Human papillomavirus in oral premalignant lesions’, Eur J Cancer B Oral Oncol, 32B(4), pp. 264-70.
O’Sullivan, E. M. (2011) ‘Prevalence of oral mucosal abnormalities in addiction treatment centre residents in Southern Ireland’, Oral Oncol, 47(5), pp. 395-9.
PINDBORG, J. J., RENSTRUP, G., POULSEN, H. E. and SILVERMAN, S. (1963) ‘STUDIES IN ORAL LEUKOPLAKIAS. V. CLINICAL AND HISTOLOGIC SIGNS OF MALIGNANCY’, Acta Odontol Scand, 21, pp. 407-14.
Reichart, P. A. and Philipsen, H. P. (2005) ‘Oral erythroplakia–a review’, Oral Oncol, 41(6), pp. 551-61.
Roza, A. L. O. C., Kowalski, L. P., William, W. N., de Castro, G., Chaves, A. L. F., Araújo, A. L. D., Ribeiro, A. C. P., Brandão, T. B., Lopes, M. A., Vargas, P. A., Santos-Silva, A. R. and Cancer, L. A. C. O. G. B. G. o. H. a. N. (2021) ‘Oral leukoplakia and erythroplakia in young patients: a systematic review’, Oral Surg Oral Med Oral Pathol Oral Radiol, 131(1), pp. 73-84.
Shafer, W. G. and Waldron, C. A. (1975) ‘Erythroplakia of the oral cavity’, Cancer, 36(3), pp. 1021-8.
Shear, M. (1972) ‘Erythroplakia of the mouth’, Int Dent J, 22(4), pp. 460-73.
Thavarajah, R., Rao, A., Raman, U., Rajasekaran, S. T., Joshua, E., R, H. and Kannan, R. (2006) ‘Oral lesions of 500 habitual psychoactive substance users in Chennai, India’, Arch Oral Biol, 51(6), pp. 512-9.
Villa, A., Villa, C. and Abati, S. (2011) ‘Oral cancer and oral erythroplakia: an update and implication for clinicians’, Aust Dent J, 56(3), pp. 253-6.
Warnakulasuriya, S. (2018) ‘Clinical features and presentation of oral potentially malignant disorders’, Oral Surg Oral Med Oral Pathol Oral Radiol, 125(6), pp. 582-590.
Yang, S. W., Lee, Y. S., Chang, L. C., Hsieh, T. Y. and Chen, T. A. (2015a) ‘Outcome of excision of oral erythroplakia’, Br J Oral Maxillofac Surg, 53(2), pp. 142-7.
Yang, S. W., Lee, Y. S., Chang, L. C., Hwang, C. C., Luo, C. M. and Chen, T. A. (2015b) ‘Clinical characteristics of narrow-band imaging of oral erythroplakia and its correlation with pathology’, BMC Cancer, 15, pp. 406.
Zaini, Z. M., McParland, H., Møller, H., Husband, K. and Odell, E. W. (2018) ‘Predicting malignant progression in clinically high-risk lesions by DNA ploidy analysis and dysplasia grading’, Sci Rep, 8(1), pp. 15874.







